Abstract
Introduction
In chronic lymphocytic leukemia (CLL), TP53 aberration (TP53ab: del(17p) and/or TP53 mutation) remains the only biomarker directly influencing clinical practice (Blood. 2018;131(25):2745-2760). Further, TP53abs are early events that can lead to acquisition of additional somatic mutations, which suggest genomic instability caused by loss of p53, especially upon therapy. Single-agent ibrutinib is an effective therapy for both treatment-naïve (TN) and relapsed or refractory (R/R) CLL patients including those with del(17p). However, long-term progression-free survival (PFS) and overall survival are inferior for ibrutinib treated patients with TP53abs and R/R CLL due to early (<2 years) development of Richter's transformation or late (≥2 years) acquired resistance mutations in BTK and PLCG2.
Aim
To assess PFS and time to progression (TTP) in CLL patients treated with ibrutinib based on number of TP53abs.
Methods
Twenty-nine TP53 aberrated CLL patients from a phase II study of single-agent ibrutinib were included (Lancet Onc. 2015;16(2):169-176, www.clinicaltrials.gov #NCT01500733): 21 TN and 8 with R/R CLL. All patients received ibrutinib 420 mg once daily until progressive disease (PD) or intolerable side effects. The study was approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
The TP53 gene (exons 2-10) was assessed from baseline patient samples by deep targeted Next Generation Sequencing. Library preparation was performed according to manufacturer's protocol (Nimblegen) and pooled libraries were sequenced as paired-end on a NextSeq (2x150 base PE, Illumina). A bioinformatic pipeline was developed in CLC Biomedical Genomics Workbench 3.0 (Qiagen) including a validated dilution step resulting in a limit of detection of 0.2% variant allelic frequency (VAF). Synonymous mutations and single nucleotide polymorphisms were excluded.
Patients were followed from treatment initiation until death, PD or end of follow-up, whichever came first. Pairwise log-rank test was applied for PFS, while Aalen-Johansen estimates of cumulative incidence rates were applied for TTP considering death as a competing risk. Analyses downstream of CLC were performed with R version 3.4.1.
Results
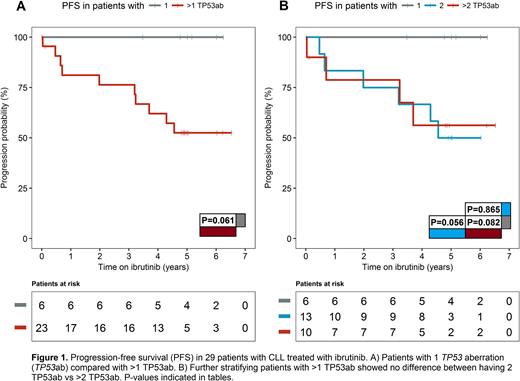
Twenty-four of 29 (83%) patients harbored 124 TP53 mutations (TP53muts) with a median of 0.7% VAF (IQR 0.3-3.1%): 22 high burden (VAF >10%) and 102 low burden TP53muts (VAF ≤10%) including 67 minor TP53muts (VAF <1%). Missense mutations accounted for 73%, while 14% of mutations were located to splice sites and another 14% were indels including 8 frameshift, 7 nonsense, and 2 in-frame mutations. Ninety-four percent of mutations were located within exons 5-9. Twenty-two patients had both del(17p) and TP53mut, 5 had del(17p) only, and 2 patients had only TP53muts of whom 1 had a single TP53:c.847C>T, which was predicted to encode functional p53 (http://p53.iarc.fr). Based on the number of TP53abs, 6 patients had only 1 TP53ab and 23 had >1 TP53ab. All patients with >1 TP53ab had biallelic TP53 disruption including 1 patient harboring a total of 38 TP53muts without concomitant del(17p).
With a median follow-up time of 5.0 years (IQR: 4.9-6.0), PFS was 100% for patients with only 1 TP53ab. Among the 23 patients with >1 TP53ab, 9 patients had PD and 1 patient had died without progression (Figure 1A). There was no difference in PFS between patients with 2 TP53ab and patients with >2 TP53ab (Figure 1B). The cumulative incidence of PD for patients with >1 TP53ab was 39%, while no patients with only 1 TP53ab had progressed. Even though 5/6 patients with only 1 TP53ab were also TN, having >1 TP53ab impacted PFS negatively among both patients with TN (4 with PD and 1 death) and R/R CLL (5 with PD).
Conclusion
Patients with monoallelic TP53 aberration demonstrate excellent outcome on single-agent ibrutinib regardless of prior treatment status. Biallelic TP53 aberration identifies patients who have inferior outcome with ibrutinib monotherapy and may benefit from combination therapy. Among patients with biallelic TP53 disruption, having more than two TP53 aberrations does not portend a worse prognosis. The prognostic role of biallelic TP53 aberration calls for validation in independent, prospective studies and in clinical trials using targeted combination regimens.
Brieghel:Rigshospitalet, Denmark: Research Funding; Arvid Nilson's Fund: Research Funding. Wiestner:Pharmacyclics LLC, an AbbVie Company: Research Funding. Niemann:Janssen: Consultancy, Research Funding; Novo Nordisk Foundation: Research Funding; Gilead: Consultancy; CSL Behring: Consultancy; Danish Cancer Society: Research Funding; Roche: Consultancy; AstraZeneca: Consultancy; Abbvie: Consultancy, Research Funding; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal